CHAPTER 2 SOLUTION
Q
– 2.1 Define the term solution. How many types of solution are formed? Write
briefly about each type with an example.
Ans. A solution is a homogeneous mixture of two or more chemically non-reacting substances whose composition can be varied within creation limits.
Types of solution
A solution can be solid, liquid or a gas depending upon the physical state of
the solvent.
|
S.No |
Solute |
Solvent |
Type of Sol. |
Examples |
|
SOLID 1. 2. 3. LIQUID 4. 5. 6. GASEOUS 7. 8. 9. |
SOLUTION Solid Liquid Gas SOLUTION Solid Liquid Gas SOLUTION Solid Liquid Gas |
(SOLID Solid Solid Solid (LIQUID Liquid Liquid Liquid (GASEOUS Gas Gas Gas |
SOLVENT) Solid in Solid Liquid in Solid Gas in Solid SOLVENT) Solid in Liquid Liquid in Solid Gas in Liquid SOLVENT) Solid in Gas Liquid in Gas Gas in Gas |
Alloys (brass, German silver, bronze,
22 carat gold etc.) Hydrated salts, Amalgam of Hg with Na Dissolved gases in minerals or H2 in
Pd Salt of glucose of sugar urea
solution in water Methanol of ethanol in water Aerated drinks O2 in
water Iodine vapours in air, camphor in N2 gas Humidity in air, chloroform mixed
with N2 gas Air (O2 + N2) |
Q
– 2.2. Suppose a solid solution is formed between tow substance, one whose
particles are very large and the other whose particles are very small. What
type of this solid solution is likely to be?
Ans. Interstitial solid solution.
Ans. (i) Mole fraction – mole fraction of a constituent (solute as well as solvent) is the fraction obtained by dividing number of moles of that constituent by the total number of moles of all the constituents present in the solution.
(ii) Molality- Molality of a solution is defined as the number of moles of the
solute dissolved in 1000 grams (1 kg) of the solvent.
(iii) Molarity – Molarity of a solution is defined as the number of moles of
the solute dissolved per liter (or dm3) of solution.
(iv) Mass percentage – Mass percentage is one way of representing the
concentration of an element in a compound or a component in a mixture. Mass
percentage is calculated as the mass of a component divided by the total mass
of the mixture, multiplied by 100%.
Mass of nitric acid = 68 g, Mass of solution = 100 g
Molar mass of HNO3 = 63 g mol-1

Q – 2.5. A solution of glucose in water is labelled as 10% w/w. What would be the molality and mole fraction of each component in the solution? If the density of the solution is 1.2 g ml-1, then what shall be the molarity of the solution?

Q
– 2.6. How many ml of a 0.1 M HCL are required to react completely with 1 g
mixture of Na2CO3 and NaHCO3 containing equimolar amounts of the two?



Q – 2.8. An antifreeze solution is prepared from 222.6 g of ethylene glycol, C2H4(OH)2 and 200g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g ml-1, then what shall be the molarity of the solution?
Ans. Mass of the solute, C2H4(OH)2 =222.6 g,
Molar mass of C2H4(OH)2 = 62 g mol-1

Q
– 2.9. A sample of drinking water was found to be severely contaminated with
chloroform, CHCL3, supposed to be carcinogen. The level of contamination was 15
ppm (by mass). (i) Express this in percent by mass. (ii) Determine the molality
of chloroform in the water sample.

Q
– 2.10. What role does the molecular interaction play in solution of alcohol
and water?

Q – 2.12. State Henry’s law and mention some of its important applications.
Ans. Henry’s law – This is the most important factor influencing the solubility of a gas in a liquid at a particular temperature. A little thought clearly reveals that as we compress the gas over the liquid (i.e., we increase the pressure), the solubility will increase.
Quantitatively, the effect of pressure on the solubility of a gas in a liquid
was studied by Henry (in 1803) and is called Henry’s law.
Applications of Henry’s law :
(i) in the production of carbonated beverages.
(ii) in the deep sea diving.
(iii) in the function of lungs.
(iv) For climbers or people living at high altitudes.
Q – 2.13. The partial pressure of ethane over a saturated solution containing 6.56 × 10-2 g of ethane is 1 bar. If the solution contains 5.00 × 10-2 g of ethane, then what shall be the partial pressure of the gas?

Q
– 2.14. What is meant by positive and negative deviations from Raoult’s law and
how is the sign of ∆_solH related to positive and negative deviations from
Raoult’s law ?
(b) Non- ideal solutions showing negative deviations – if for the two
components A and B, the forces of interaction between the A and B molecules are
more than the A – A and B – B forces of interaction, the escaping tendency of A
and B types of molecules from the solution becomes less than from the pure
liquids. In other words, for any composition of the solution, the partial
vapour pressure of the solution will also be less than that expected from
Raoult’s law.
Q – 2.15. An aqueous solution of 2% non- volatile solute exerts a pressure of 1.0041 bar at the normal boiling point of the solvent. What id solvent. What is the molecular mass of the solute?
Ans. Vapor pressure of pure water at the boiling (p0) = 1 atm = 1.013 bar
Vapor pressure of solution ( ps) = 1.004 bar; Mass of solute (w2) = 2g
Mass of solution = 100 g ; Mass of solvent = 98 g
Q – 2.16. Heptane and octane from ideal solution. At 373 K, the vapour pressure of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35.0 g of octane?

Q – 2.17. The vapour pressure of water is 12.3 kPa at 300 K. Calculate the vapor of 1 molal solution of a solute in it.

Q – 2.18. Calculate the mass of a non-volatile solute (Molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour to 80 %.
Ans. Reduction of vapour pressure to 80% means that is P0 = 100 mm,
than Ps = 80 mm.
Applying complete formula
Note that complete formula is required because concentration of solution is greater
than 5%. Complete formula can also be applied in the from.


Q – 2.19. A solution containing 30 g of a non – volatile solute exactly in 90 g water has a vapour pressure of 2.8 kPa at 298 K. Further 18 g of water is then added to the solution, the new vapour pressure becomes 2.9 kPa at 298 K. Calculate
(i) molar mass of the solute. (ii) vapour pressure of
water at 298 K.

Q
– 2.20. A 5% solution (by mass) of cane sugar in water has freezing point of
271 K. Calculate the freezing point of a 5% glucose in water if freezing point
of pure water is 273.15 K.
Ans.
5% solution by mass means 5 g of solute is present in 100 g of solution
؞
Mass of solvent (water) = 95 g
Q – 2.21. Two elements A and B from compounds having molecular formula AB2 and BA4 . when dissolved in 20 g of benzene (C6H6), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. the molal depression constant for benzene is 5.1 K kg mol-1. Calculate atomic masses of A and B.

Suppose atomic masses of
A and B are ‘a’ and ‘b’ respectively. Then
Molar mass of AB4 = a + 2b = 110.87 g mol-1 (i)
Molar mass of AB4 = a + 4b = 196.15 g mol-1 (ii)
Eqn. (ii) – Eqn. (i) gives 2 b = 85.28 or b = 42.64
Substituting in eqn. (i), we get a + 2 × 42.64 = 110.87 or a = 25.59
Thus, Atomic mass of A = 25.59 u,
Atomic mass of B = 42.64 u
Q – 2.22. At 300 K, 36 g glucose present per litre in its solution has an osmatic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bar at the same temperature, what should be its concentration?

Q
– 2.23. Suggest the most important type of intermolecular interaction in the
following pairs:
(i) n-hexane and n-octane (ii) I2 and CCI4 (ii) NaClO4 and water (iv) methanol
and
acetone (v) acetonitrile (CH3CN) and acetone (C3H6O).
Ans. (i) both are non-polar. Hence, intermolecular interaction in them will be London dispersion forces (Discussed in class XI).
(ii) Same as (i).
(iii) NaClO4 gives Na+ and CiO-4 ions in the solution while water is a polar
molecule. Hence, intermolecular interaction in them will be ion-dipole
interactions.
(iv) Both are polar molecules. Hence, interactions in them will be
dipole-dipole interactions.
(v) Same as (iv).
Q – 2.24. Based on solute- solvent interaction, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN
Ans. (i) Cyclohexane and n-octane both are non- polar. Hence, they mix completely in all
proportions.
(ii) KCl is an ionic compound while n-octane is non-polar. Hence, KCl will be
dissolve at
all in n-octane.
(iii) CH3OH and CH3CN both are polar but CH3CN is less polar than CH3OH. As the
solvent is non-polar, CH3CN will dissolve more than CH3OH in n-octane.
Thus, the order of solubility will be KCl < CH3OH< CH3CN<Cyclohexane.
(ii) Insoluble because toluene is non-polar.
(iii) Highly soluble because formic acid can form hydrogen bonds with water.
(iv) Highly soluble because ethylene glycol can form hydrogen bonds with water.
(v) Insoluble because chloroform is an organic liquid.
(vi) Partially soluble because –OH group is polar but the large hydrogen part
(C5H11) is
non-polar.

Q – 2.27. If the solubility product of CuS is 6 × 10-16, calculate the maximum molarity of CuS in aqueous solution.
Ans. Maximum molarity of CuS is aqueous solution = solubility of CuS in mol-1
If S is the solubility of CuS in mol-1, then
Q
– 2.28. Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile
(CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN.
Q – 2.29. Nalorphene (C19H21NO3), similar to morphine, is used to combat withdrawal symptoms in narcotic user. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 × 10-3 m aqueous solution required for the above dose.
Molar mass of C19H21NO3 = 19 × 12 + 21 +14 + 48 =311 g mol-1
؞ 1.5 × 10-3
mole of C19H21NO3 = 1.5 × 10-3 × 311 g = 0.467 g = 467 mg
؞
Mass of solution = 1000 g + 0.467 g = 1000.467g
Q –
2.30. Calculate the amount of benzoic acid (C6H5COOH) required for preparing
250 ml of 0.15 M solution in methanol.
Ans. 0.15 M solution means that 0.15 mole of benzoic acid is present in 1 L,
i.e., 1000 ml of the solution.
Molar mass of benzoic acid (C6H5COOH) = 72 + 5 + 12 + 32 + 1 = 122 g mol-1
؞ 0.15
mole of benzoic acid = 0.15 × 122 g = 18.3 g
؞
Thus, 1000 ml of the solution contain benzoic acid = 18.3 g
Q –
2.31. The depression in freezing point of water observed for the same amount of
acetic acid, trichloroacetic acid trifluoroacetic acid increase in the order
given above. Explain briefly.
acetic acid < trichloroacetic acid < trifloroacetic acid

Fluorine, being most electronegative, has the highest electron withdrawing inductive effect. Consequently, trifluoroacetic acid is the strongest aid while acetic acid is the weakest acid. Hence, trifluoroacetic acid ionizes to the largest extent while acetic acid ionizes to the minimum extent to give ions in their solutions in water. Greater the ions produced, grater is the depression in freezing point. Hence, the depression in freezing point is maximum for the fluoroacetic acid and minimum for acetic acid.


Q – 2.33. 19.5 g of CH2FCOOH is dissolved in 500 g of water. the depression in the freezing point observed is 1.00C. Calculate the van’t Hoff factor and dissociation constant of floroacteic acid. Kƒ for water is 1.86 K kg mol
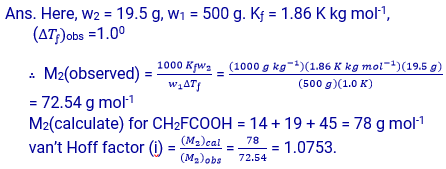
Calculation of dissociation constant. Suppose degree of dissociation at the given
concentration is α.

Q – 2.34. Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapour pressure of water at 293 K when 25 g glucose is dissolved in 450 g of water.
Ans. Here, P0 = 17.535 mm, w2 = 25 g, w1 = 450 g
For solute (glucose, C6H12O6), M2 = 180 g mol-1,
Q –
2.35. Henry’s law constant for the molality of methane in benzene at 298 K is
4.27 × 105 mm Hg. Calculate the solubility of methane in benzene at 298 K under
760 mm Hg.

Q – 2.36. 100 g liquid A (molar mass 140 g mol-1) was dissolved in 1000 g of liquid B (molar mass 180 g mol-1 ). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 torr.

Substituting this value in eqn. (i), we get PA = 0.114 × 280.7 torr = 32 torr.
100 × xacetone 0
11.8 23.4
36.0 50.8 58.2
64.5
72.1
Pacetone/mm Hg 0
54.9 110.1 202.4
322.7 405.9
454.1 521.1
Pchloroform/mm Hg 632.8 548.1
469.4 359.7 257.7
193.6 161.2
120.7
Polt this data also on the same graph paper,Indicate whether it has positive
deviation or negative deviation from the ideal solution.
Ans.
xacetone 0
11.8 23.4
36.0 50.8
58.2 64.5 72.1
Pacetone/mm Hg 0
54.9 110.1 202.4
322.7 405.9
454.1 521.1
Pchloroform/mm Hg 632.8 548.1
469.4 359.7 257.7
193.6 161.2 120.7
PTotal
632.8 603.0
579.5 562.1 580.4
599.5 615.3 641.8
Molar mass of toluene C6H5CH3) = 92 g mol-1
Applying Raoult’s law,
PBenzene = xBenzene × P0Benzene = 0.486×50.71 mm = 24.65 mm
PTotal = xToluene × P0Toluene = 0.514 × 32.06 mm = 16.48 mm
؞ Mole fraction of benzene in law vapoure phase

Q – 2.39. The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. water is in equilibrium with air at a pressure of 10 atm. At 298 K, if the Henry’s law constants for oxygen and nitrogen are 3.30 × 107 mm and 6.51 × 107 mm respectively, calculate the composition of these gases in water.
As air contains 20% oxygen and 79% nitrogen by volume,
Q – 2.40. Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmatic pressure is 0.75 atm at 270C.

Molar mass of CaCl2 =40 +
2 × 35.5 = 111 g mol-1
؞
Amount dissolved = 0.0308 × 111 g = 3.42 g.
Volume of solution = 2 L, T = 250C =298 K
As K2SO4 dissociates completely as K2SO4 → 2K+ + SO42- ,
i.e., ions produced = 3 , ؞ I =
3
